I have always lusted after his patinas (and work) after seeing it up close when I took his chasing class oh so many years ago. As soon as I saw this posted, I signed up. I was the 9th out of 10 students, and then I had to wait almost 6 months for the workshop.
So last Friday I flew to Mesa with my copper tiles, apron, book for notes, safety glasses and expectations. The workshop started on Saturday, for 3 day and I flew home on Tuesday.
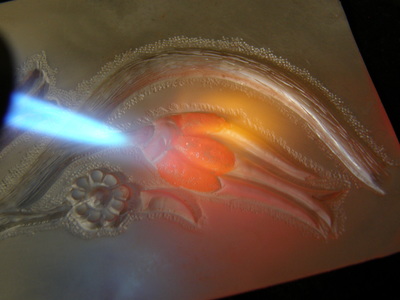
It was a wonderful workshop. David gives great demonstrations and shares an amazing amount of information.
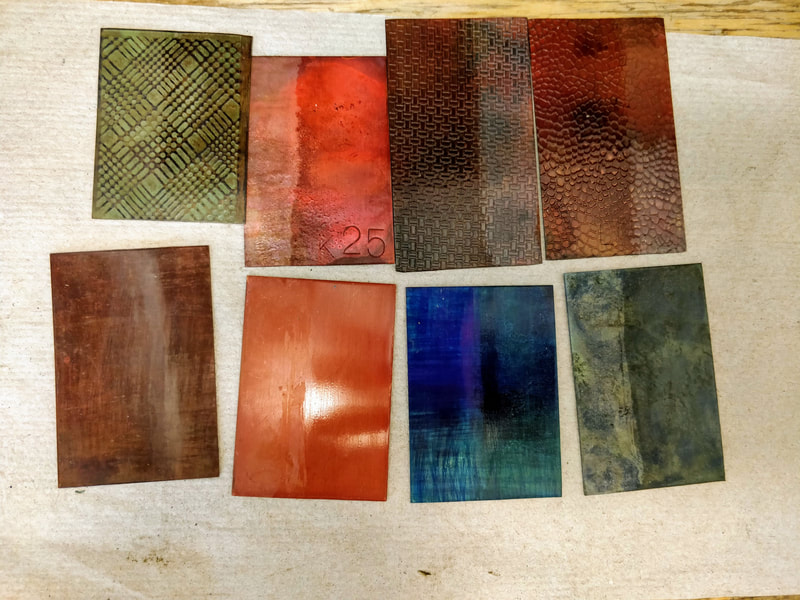
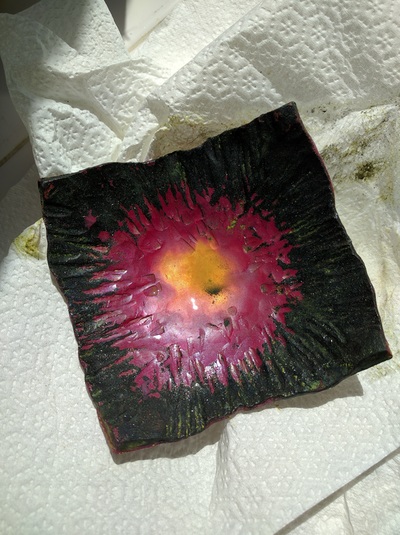
We covered cold patinas, hot patinas, boiled patinas, and gold leaf along with layering; we only worked on copper but there is also brass, bronze, steel and even silver that patinas can be applied to; nor did we cover all the possible chemicals that can be used - we covered just the ones David uses most often.
Of course Bunny Bravehart came with me but he just watched as he was afraid of getting burned.
The day went like this.
- David gives us a recipe and then he mixes it and put the solution in jars for each station
- David then demonstrates the application (creation) of the patina
- Students ask questions
- Student apply patina to their copper tiles
- Students share results with each other and ask David more questions
- Repeat
What we used: But not how we used them as that is what a class or research is for!
- Cupric Nitrate
- Ferric Nitrate
- Ammonia
- Sodium Chloride (Salt)
- Dihydrogen monoxide (Water. Yes it is a chemical and a powerful solvent; hey it can kill you!!)
- Bismuth Nitrate
- Ammonium Chloride
- Cobalt Nitrate
- Copper Sulfate
- Silver Nitrate
- Gun Bluing
- Liver of Sulfur
- Gold Leaf























































































































































 RSS Feed
RSS Feed
